Oncologists' reflections on patient rights and access to compassionate use drugs: A qualitative interview study from an academic cancer center | PLOS ONE

Manufacturer's Compassionate Use Policies: Companies with Posted Policies More Than Doubled Since September 2016

Proportion of Gaucher Patients per country receiving their treatment on... | Download Scientific Diagram

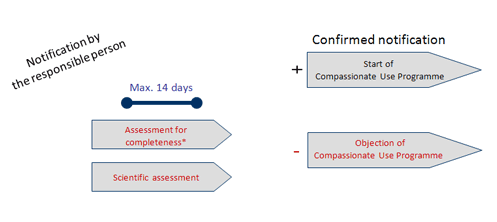
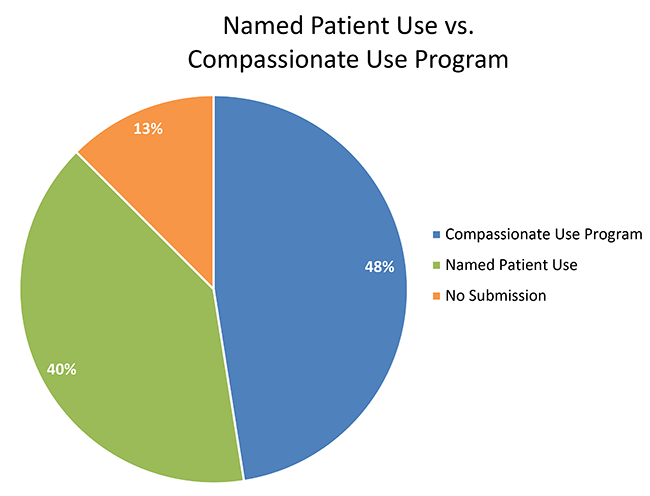
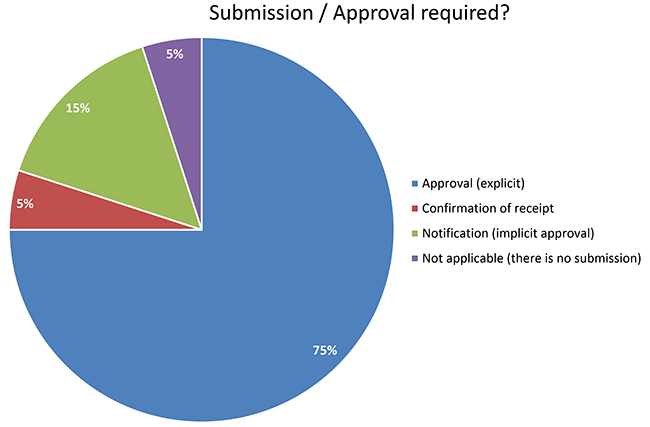
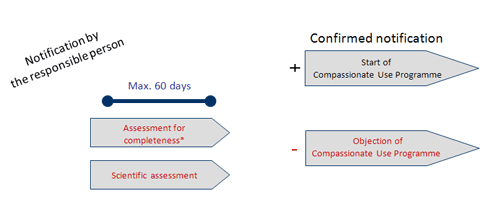
Compassionate use programs and the European regulatory system Filip Josephson M.D., Ph.D. Clinical Assessor. - ppt download

Understanding the challenges and ethical aspects of compassionate use of drugs in emergency situations Goyal PK, Mathur R, Medhi B - Indian J Pharmacol